Gemmological and Spectroscopic Characteristics of Natural and Synthetic Fluorites
-
摘要:
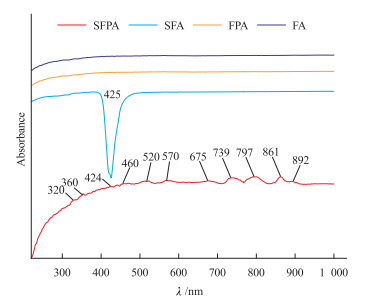
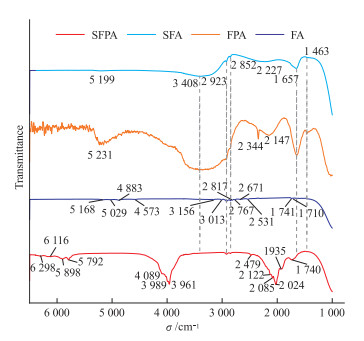
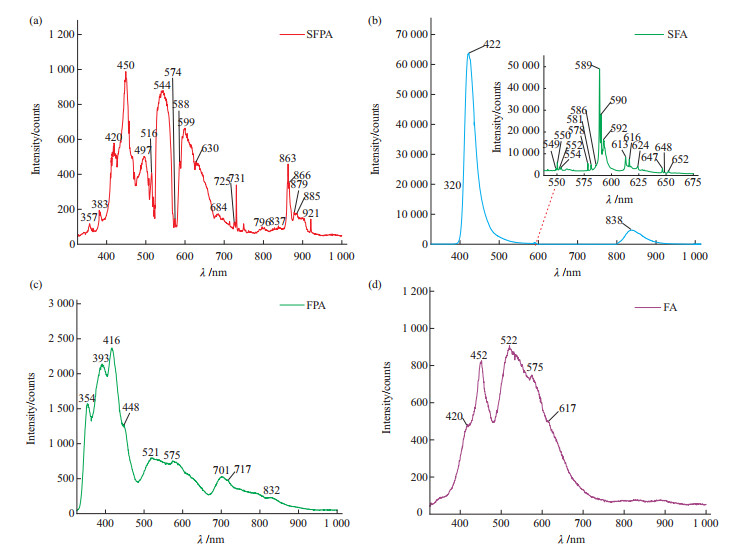
萤石既是一种重要的工业矿物,也是珠宝、矿物晶体市场的重要品种。近年来,随着宝石首饰市场需求的多样化,萤石也被广泛用于制作刻面宝石、手串、手镯等珠宝饰品。着手于天然及合成萤石的鉴定需求,本文对天然萤石及合成萤石样品进行显微镜观察、飞秒激光剥蚀等离子体质谱(fs-LA-ICP-MS)、X射线荧光光谱(XRF)、紫外-可见吸收光谱(UV-Vis)、红外光谱(FTIR)及光致发光光谱(PL)测试,获取了不同样品的包裹体、光学性质、化学成分及谱学特征。结果表明:(1)合成萤石中无明显包裹体,而天然萤石中有大量气液包裹体;(2)合成紫色及无色萤石中掺杂的Nd及Eu元素的质量分数分别达775.13×10-6~832.63×10-6和15 173.88×10-6~16 674.97×10-6,显著高于天然萤石,且可以被XRF检出而易于鉴别;(3)合成萤石的紫外-可见光谱中可以显著观察到由掺杂的稀土元素导致的吸收峰和荧光峰,而天然萤石的紫外-可见光谱并无明显的吸收峰或荧光峰;(4)红外光谱显示,天然萤石样品中有大量的CO32-、CO2、羟基和水相关的振动峰,而合成萤石则没有,是区分萤石是否天然的重要特征;(5)光致发光光谱则表明,合成萤石因各稀土元素含量均较天然萤石高,不同价态的多种稀土元素均会产生荧光峰,导致其光谱较天然萤石复杂,且合成无色萤石掺杂大量Eu, Eu跃迁峰强度显著高于天然萤石,可用于对萤石天然性的辅助鉴别。
Abstract:Fluorite is not only an important industrial mineral, but also a major species in the jewelry and mineral collection market. In recent years, with the diversification of demand in the gemstone jewelry market, fluorite has been widely used to make faceted gemstones, beads, bracelets, and other forms of jewelry. Fluorite is a common and easy to be synthesized material. Synthetic fluorite sometimes appears in the mineral collections and gem markets. Based on the identification demand of natural and synthetic fluorite, through microscopic observation, femtosecond laser ablation plasma mass spectrometer (fs-LA-ICP-MS), X-ray fluorescence spectrometer (XRF), ultraviolet-visible absorption spectrometer (UV-Vis), infrared spectrometer (FTIR), and photoluminescence spectrometer (PL), inclusion, optical properties, chemical compositions, and spectroscopy characteristics of natural fluorite and synthetic fluorite samples were obtained. Microscopic observation shows that there are no obvious inclusions in synthetic fluorite samples, while natural fluorite samples contain a large amount of gas-liquid inclusions. By fs-LA-ICP-MS, the Nd and Eu contents in synthetic violet fluorite and colourless fluorite samples reach 775.13×10-6-832.63×10-6 and 15 173.88×10-6-16 674.97×10-6, respectively, significantly higher than those in natural fluorite samples, such amount of Nd and Eu can be detected by XRF and makes it easy for identification. In UV-Vis spectra, synthetic fluorite samples shows obvious absorption and fluorescence peaks caused by doped REE, while natural fluorite samples show nothing obvious in their spectra. FTIR shows a large number of vibration peaks related to CO32-, CO2, hydroxyl groups, and water in natural fluorite samples, while synthetic fluorite samples do not, which is a useful character to distinguish whether fluorite is natural or not. The PL spectrum indicates that synthetic fluorite has a higher content of various REE than that in natural fluorite, and multiple REE in different valence states produce different fluorescence peaks, resulting in a more complex PL spectrum than that in natural fluorite. Moreover, the Eu2+ transition peak intensity of synthetic colorless fluorite samples doped with a large amount of Eu is significantly higher than that of natural fluorite, which can be used to assist in the identification of fluorite's naturalness.
-
Keywords:
- synthetic fluorite /
- REE doping /
- fs-LA-ICP-MS /
- UV-Vis /
- FTIR /
- PL
-
-
表 1 天然和合成萤石样品的常规宝石学特征
Table 1 Conventional gemmological characteristics of natural and synthetic fluorite samples
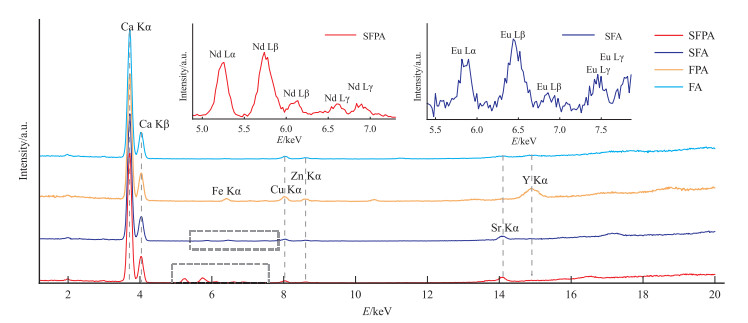
样品编号 颜色 重量/ct 折射率 相对密度 长波紫外光下荧光 短波紫外光下荧光 消光现象 SFPB 紫色 12.20 1.434 3.23 弱蓝紫色 无 全暗 SFB 无色 12.11 1.432 3.20 强蓝白色 强蓝白色 全暗 FPB 紫色 17.35 1.432 3.22 弱蓝紫色 无 异常消光 FB 无色 15.72 1.431 3.22 弱白色 无 异常消光 表 2 合成萤石和天然萤石样品的微量元素含量
Table 2 Contents of trace elements of synthetic fluorite samples and natural fluorite samples
×10-6 样品编号 Na Mg Al Si K Ga Ge As Sr La Ce Pr Nd Sm Eu SFA-1 413.79 61.72 17.48 1 571.08 16.15 - - - 107.68 3.86 6.67 0.83 4.08 0.93 829.39 SFA-2 106.74 27.93 5.77 1 229.87 - 0.01 - 0.71 105.58 3.77 6.61 0.78 3.98 0.90 799.84 SFA-3 72.36 33.53 6.62 1 132.80 0.99 0.07 0.69 - 101.78 3.69 6.08 0.79 4.02 0.94 818.13 SFA-4 53.49 20.84 9.69 1 222.98 - - 0.01 - 105.97 3.71 6.47 0.80 3.84 0.93 832.63 SFA-5 67.79 14.29 4.48 1 104.69 - 0.04 0.05 0.47 99.91 3.48 6.17 0.78 3.79 0.97 831.88 SFA-6 470.29 110.29 33.58 2 812.78 9.96 0.03 - 0.38 100.41 3.51 5.92 0.75 3.79 0.93 800.87 SFA-7 476.05 109.16 33.54 2 195.70 21.49 - 0.76 - 95.43 3.50 6.16 0.73 3.73 1.05 794.68 SFA-8 322.23 83.07 26.75 1 856.62 13.23 - 0.10 - 94.58 3.31 5.83 0.76 3.72 1.05 797.28 SFA-9 440.17 110.77 30.65 2 379.68 12.94 0.05 - 0.65 102.26 3.50 5.95 0.78 3.59 1.03 775.13 SFA-10 762.84 186.29 48.09 3 264.27 16.46 0.04 - - 97.18 3.59 6.10 0.77 3.80 0.92 784.13 SFPA-1 - 4.19 0.90 895.46 - 48.99 113.45 75.78 133.30 7.56 13.69 2.49 15 461.72 1.21 0.41 SFPA-2 - 3.84 - 781.46 - 49.71 114.32 71.71 135.73 7.16 13.32 2.61 16 221.28 1.13 0.31 SFPA-3 8.67 2.32 - 401.44 - 47.57 106.81 74.14 133.09 6.80 13.14 2.55 15 173.88 1.11 2.19 SFPA-4 - 3.66 0.10 564.06 - 48.94 113.72 76.00 134.08 6.79 13.06 2.49 15 703.65 1.16 0.31 SFPA-5 - 3.02 - 229.56 - 46.58 113.61 75.06 132.12 7.21 13.79 2.48 15 756.14 1.03 0.36 SFPA-6 21.30 3.10 0.06 892.70 9.84 47.93 103.53 66.58 130.00 7.14 13.46 2.42 16 526.04 1.14 0.33 SFPA-7 - 1.54 - 527.29 - 48.53 103.77 72.44 128.96 7.36 13.53 2.51 16 211.71 1.26 0.30 SFPA-8 20.34 3.07 - 456.11 - 48.35 108.17 69.92 127.80 7.07 13.34 2.59 16 438.53 1.06 0.31 SFPA-9 - 2.69 1.67 392.68 - 49.98 115.50 72.08 131.80 7.11 13.42 2.57 16 674.97 1.31 0.28 SFPA-10 - 3.02 - 327.00 - 49.39 106.73 72.77 127.03 6.70 12.67 2.57 15 405.02 1.14 0.31 FA-1 321.54 0.62 3.90 465.82 9.35 0.06 - 0.64 7.83 0.02 0.01 - 0.01 0.01 - FA-2 - 2.29 - 387.39 2.57 - - 1.52 8.49 0.21 0.12 0.03 0.22 0.10 0.03 FA-3 22.66 - 4.30 266.35 5.55 0.02 - 0.13 7.91 0.11 0.08 0.02 0.16 0.07 0.02 FA-4 37.54 3.05 - 923.25 - 0.07 0.62 0.33 8.18 0.06 0.03 0.01 0.10 0.08 0.01 FA-5 116.05 1.04 - 7.94 25.04 - - - 7.94 0.01 - - 0.01 0.01 - FA-6 108.38 - - - 28.84 0.06 1.16 0.09 8.08 0.25 0.19 0.04 0.35 0.14 0.05 FA-7 27.59 3.92 0.82 - - - 0.11 - 9.41 0.02 0.02 - 0.02 0.03 0.01 FA-8 - - - 1 905.02 0.66 - - - 8.63 0.03 0.03 0.01 0.07 0.04 0.01 FA-9 - 0.62 2.33 - 8.79 0.07 - 0.37 7.94 0.15 0.11 0.03 0.19 0.12 0.04 FA-10 - 3.15 2.25 - 5.62 - 0.24 0.92 8.75 0.17 0.14 0.03 0.19 0.10 0.05 FPA-1 - - - 1 013.90 0.70 0.07 0.18 0.09 80.82 2.36 3.97 0.46 1.67 0.44 0.08 FPA-2 - 1.17 - 361.72 - 0.04 0.17 0.69 87.71 1.34 2.27 0.23 1.04 0.23 0.06 FPA-3 - 1.16 0.55 876.27 - - 1.03 - 86.43 2.07 3.65 0.43 1.69 0.47 0.14 FPA-4 10.20 - 0.38 253.75 - - 0.81 - 88.89 2.45 4.09 0.48 2.00 0.44 0.10 FPA-5 63.69 7.16 - - - - - 0.82 87.92 5.06 8.42 1.01 3.72 0.81 0.19 FPA-6 - - 3.38 - 1.03 - 0.36 - 72.77 2.30 3.74 0.40 1.57 0.36 0.09 FPA-7 - 1.75 - - 4.01 - 0.77 1.40 93.52 0.33 0.72 0.13 0.63 0.53 0.23 FPA-8 66.18 5.48 0.83 526.72 11.89 0.12 1.04 - 64.70 5.29 8.52 0.96 3.68 0.66 0.13 FPA-9 - 0.80 - 1 073.19 - 0.15 0.22 0.95 75.65 1.68 2.70 0.30 1.28 0.28 0.05 FPA-10 36.65 3.58 2.85 - 4.42 0.03 - 0.01 78.07 3.06 5.02 0.58 2.13 0.43 0.08 注:“-”表示低于检测限 -
[1] He J F, Chen H, Zhang M M, et al. Combined inhibitors of Fe3+, Cu2+ or Al3+ and sodium silicate on the flotation of fluorite and quartz[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022(643): 128 702.
[2] Crowther J. Ultraviolet fluorescence photography-Choosing the correct filters for imaging[J]. Journal of Imaging, 2022: 8(6): 162. doi: 10.3390/jimaging8060162
[3] 孙益坚, 张宇, 柯于球. 萤石矿物LA-ICP-MS微区分析标准物质研制及激光剥蚀行为调控[C]//第十八届全国稀土分析测试学术研讨会. 秭归, 2021. Sun Y J, Zhang Y, Ke Y Q. Development of LA-ICP-MS standard material and control and adjustment of laser ablation behavior for micro area analysis of fluorite[C]// The 18th National Symposium on Rare Earth Analysis and Testing. Zigui, 2021. (in Chinese)
[4] Sils J, Hausfeld S, Clauss W, et al. Impurities in synthetic fluorite for deep ultraviolet optical applications[J]. Journal of Applied Physics, 2009, 106(6): 063 109. doi: 10.1063/1.3224879
[5] Singh V S, Joshi C P, Moharil S V, et al. Modification of luminescence spectra of CaF2: Eu2+[J]. Luminescence, 2015, 30(7): 1 101-1 105. doi: 10.1002/bio.2865
[6] Dorenbos P. Energy of the first 4f7→4f65d transition of Eu2+ in inorganic compounds[J]. Journal of Luminescence, 2003, 104(4): 239-260. doi: 10.1016/S0022-2313(03)00078-4
[7] Pandey C, Dhopte S M, Muthal P L, et al. Eu3+ Eu2+ redox reactions in bulk and nano CaF2: Eu[J]. Radiation Effects and Defects in Solids, 2007, 162(9): 651-658. doi: 10.1080/10420150701197547
[8] Berezovskaya I V, Dotsenko V P, Voloshinovskii A S, et al. Near infrared emission of Eu2+ ions in Ca3Sc2Si3O12[J]. Chemical Physics Letters, 2013(585): 11-14.
[9] Veerasingam S, Venkatachalapathy R. Assessment of carbonate concentration and characterization of marine sediments by fourier transform infrared spectroscopy[J]. Infrared Physics & Technology, 2014(66): 136-140.
[10] Ehlert M, Radtke A, Bartmański M, et al. Evaluation of the cathodic electrodeposition effectiveness of the hydroxyapatite layer used in surface modification of Ti6Al4V-based biomaterials[J]. Materials, 2022, 15(19): 6 925. doi: 10.3390/ma15196925
[11] Kovalgin A Y, Chabert-Rocabois F, Hitchman M L, et al. A study by in situ FTIR spectroscopy of the decomposition of precursors for the MOCVD of high temperature superconductors[J]. Journal De Physique Ⅳ, 1995, 5(C5): C5-357-C5-364.
[12] Renuga Devi T S, Gayathri S. FTIR and FT-Raman spectral analysis of Paclitaxel drugs[J]. International Journal of Pharmaceutical Sciences Review and Research, 2010, 2(2): 106-110.
[13] King P L, McMillan P F, Moore G M. Infrared spectroscopy of silicate glasses with application to natural systems[M]. Ottawa: Mineralogical Association of Canada, 2004: 93-133.
[14] Patel H A, Somani R S, Bajaj H C, et al. Nanoclays for polymer nanocomposites, paints, inks, greases and cosmetics formulations, drug delivery vehicle and waste water treatment[J]. Bulletin of Materials Science, 2006(29) : 133-145.
[15] Barinov S M, Rau J V, Cesaro S N, et al. Carbonate release from carbonated hydroxyapatite in the wide temperature rage[J]. Journal Material Science Materials in Medicine, 2006, 17(7): 597-604. doi: 10.1007/s10856-006-9221-y
[16] Schott J A, Do-Thanh C, Shan W, et al. FTIR investigation of the interfacial properties and mechanisms of CO2 sorption in porous ionic liquids[J]. Green Chemical Engineering, 2021, 2(4): 392-401. doi: 10.1016/j.gce.2021.09.003
[17] Strazzulla G, Brucato J R, Palumbo M E, et al. Is it possible to detect frozen O2 and N2 on interstellar grains?[J]. Astronomy and Astrophysics, 1997, 321(2): 618-624.
[18] Varsanyi F, Dieke G H. Infrared absorption spectrum of NdCl3[J]. Journal of Chemical Physics, 1960, 33 (6): 1 616-1 618. doi: 10.1063/1.1731471
[19] Lignell H, Khriachtchev L, Lignell A, et al. Local formation of HArF in solid argon: Low-temperature limit and thermal activation[J]. Low Temperature Physics, 2010, 36 (5): 400-406. doi: 10.1063/1.3432248
[20] Giorcelli M, Bartoli M. Development of coffee biochar filler for the production of electrical conductive reinforced plastic[J]. Polymers, 2019, 11(12): 1 916. doi: 10.3390/polym11121916
[21] Fu X, Kong W, Zhang Y, et al. Novel solid-solid phase change materials with biodegradable trihydroxy surfactants for thermal energy storage[J]. RSC Advances, 2015, 5(84): 68 881- 68 889. doi: 10.1039/C5RA11842E
[22] Yang R, Li Y, Yu J. Photo-stabilization of linear low density polyethylene by inorganic nano-particles[J]. Polymer Degradation and Stability, 2005, 88(2): 168-174. doi: 10.1016/j.polymdegradstab.2003.12.005
[23] Kozłowski A, Wiszniewska J, Sikorska M. Fluid inclusions and cathodoluminescence of fluorite from carbonatites of the Tajno massif, NE Poland[J]. Mineralogical Society of Poland Special Papers, 2005(26): 40-46.
[24] Caldiño U. Energy transfer in CaF2 doped with Ce3+, Eu2+ and Mn2+ ions[J]. Journal of Physics: Condensed Matter, 2003, 15(41): 7 127-7 137. doi: 10.1088/0953-8984/15/41/020
[25] Singh B P, Parchur A K, Ningthoujam R S, et al. Enhanced photoluminescence in CaMoO4: Eu3+ by Gd3+ co-doping[J]. Dalton Transactions, 2014, 43(12): 4 779-4 789. doi: 10.1039/c3dt53408a
[26] Tao L, Zhou B, Jin W, et al. Improved multiphoton ultraviolet upconversion photoluminescence in ultrasmall core-shell nanocrystals[J]. Optics Letters, 2014, 39(21): 6 265-6 268. doi: 10.1364/OL.39.006265
[27] Serrano D, Braud A, Doualan J L, et al. Pr3+ cluster management in CaF2 by codoping with Lu3+ or Yb3+ for visible lasers and quantum down-converters[J]. Journal of the Optical Society of America B, 2012, 29(8): 1 854-1 862. doi: 10.1364/JOSAB.29.001854
[28] Zhao B, Shen D, Tan Q, et al. Morphology-controllable synthesis, energy transfer and luminescence properties of Ce3+/Tb3+/Eu3+-doped CaF2 microcrystals[J]. Journal of Materials Science, 2017(52): 5 857-5 870.
[29] Guo J, Dong Y, Li Y, et al. Structural destruction quenching of down- and upconversion emission in CaF2: Yb3+, Tb3+ [J]. Materials Research Express, 2017, 4(11): 116 201. doi: 10.1088/2053-1591/aa96c0
[30] Balabhadra S, Reid M F, Golovko V, et al. Absorption spectra, defect site distribution and upconversion excitation spectra of CaF2/SrF2/BaF2: Yb3+: Er3+ nanoparticles[J]. Journal of Alloys and Compounds, 2020(834): 155 165.
[31] Yang Y, Li W, Mei B, et al. Synthesis and enhanced upconversion luminescence upon two-wavelength excitation of Er3+: CaF2 transparent ceramics[J]. Journal of Luminescence, 2019(213): 504-509.
[32] Cantelar E, Lifante-Pedrola G, Quintanilla M, et al. Spectroscopic characterization of Er3+-doped CaF2 nanoparticles: Luminescence concentration quenching, radiation trapping and transition probabilities[J]. Journal of Alloys and Compounds, 2023(954): 170 192.
[33] Czaja M, Bodył-Gajowska S, Lisiecki R, et al. The luminescence properties of rare-earth ions in natural fluorite[J]. Phys Chem Minerals, 2012(39): 639-648.
[34] Babu B H, Billotte T, Lyu C, et al. Study of femtosecond laser writing in the bulk of Nd3+, Y3+ co-doped CaF2 crystals[J]. OSA Continuum, 2019, 2(1): 151-161. doi: 10.1364/OSAC.2.000151
[35] Gao X, Fang G, Wang Y, et al. Visible and mid-infrared spectral performances of Dy3+: CaF2 and Dy3+/ Y3+: CaF2 crystals[J]. Journal of Alloys and Compounds, 2021(856): 158 083.
[36] Zhu L, Zuo C, Luo Z, et al. Photoluminescence of Dy3+ and Sm3+: SiO2-Al2O3-LiF-CaF2 glasses[J]. Physica B: Condensed Matter, 2010, 405(21): 4 401-4 406. doi: 10.1016/j.physb.2010.07.039
[37] Danilkin M I, Belousov A P, Klimonskii S O. et al. Formation of Eu2+ and Eu3+ centers in synthesis of CaF2: Eu luminophores[J]. Journal of Applied Spectroscoscopy, 2007(74), 858-865. doi: 10.1007/s10812-007-0133-5
[38] Liu X, Li Y, Aidilibike T, et al. Pure red upconversion emission from CaF2: Yb3+/Eu3+[J]. Journal of Luminescence, 2017(185): 247-250.
[39] Boiaryntseva I A, Shiran N V, Gektin A V, et al. 1S0-luminescence in Ca1-xPrxF2+x crystals[J]. Functional Materials, 2012, 19(2): 192-196.




 下载:
下载: