Spectral Characteristic of Gem Grade Clinohumite
-
摘要:
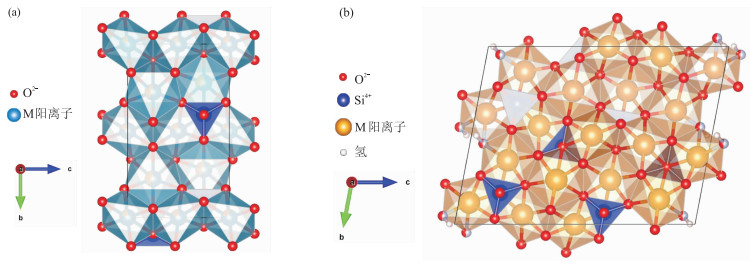
斜硅镁石是一种稀有的黄色至橙色的硅酸盐矿物,近年来市面上开始出现宝石级的斜硅镁石,而国内鲜有相关的宝石学研究。为补充该宝石的研究空白,选取三种不同色调(黄色、橙黄色和橙红色)的斜硅镁石进行常规宝石学测试,结合电子探针、激光拉曼光谱、傅里叶变换红外光谱和紫外-可见光谱分析技术,对斜硅镁石的化学成分、光谱特征及颜色成因等进行初步研究。结果表明,斜硅镁石的折射率为1.629~1.668,具有较强的双折射(DR:0.031~0.036),相对密度为3.14~3.27,短波紫外光下呈中等黄色-橙色荧光;部分样品有明显聚片双晶,包裹体形态丰富,并发现金红石包裹体;斜硅镁石含有主量元素Si、Mg和Ti,微量元素Ca、Fe、K和V,结合紫外-可见光谱推测颜色与Fe和Ti密切相关;拉曼光谱和红外光谱都显示主要峰位与SiO4四面体、MgO6八面体和-OH的振动有关,最强拉曼位移为830 cm-1和862 cm-1,最强红外光谱反射峰在925~935 cm-1附近,都与SiO4振动有关。
Abstract:Clinohumite is a rare yellow to orange silicate mineral.In recent years, gem grade clinohumite has appeared on the market, but there are few related gemmological studies in China.To supplement the research gap of this gemstone, clinohumite samples of three different colour tones (yellow, orange-yellow and orange-red) were selected for conventional gemmological tests. Combined with electronic probe, laser Raman spectrometer, Fourier transform infrared spectrometer, and UV-Vis spectrometer, the chemical compositions, spectral characteristics, and mechanism of coloration were preliminarily studied.The results show that RI of clinohumite is 1.629-1.668, with a strong birefringence(DR: 0.031-0.036).Their relative density ranges from 3.14 to 3.27, and medium yellow to orange fluorescence can be observed under shortwave ultraviolet light. Some samples have obvious twin crystal patterns and rich inclusion morphology. Rutile inclusions were found in gemstone grade clinohumite.Clinohumite contains main elements Si, Mg, and Ti, as well as trace elements Ca, Fe, K, and V. Combined with UV-Vis spectra, it is speculated that the colour is closely related to Fe and Ti.Laser Raman spectra and infrared spectra are mainly related to vibrations of SiO4 tetrahedron, MgO6 octahedron and -OH.The strongest Raman shifts (830 cm-1 and 862 cm-1) and the strongest infrared spectral reflection peaks (around 925-935 cm-1) both related to SiO4 vibration.
-
Keywords:
- clinohumite /
- infrared spectrum /
- Raman spectrum /
- UV-Vis spectrum
-
致谢: 本项目由上海市高校优秀青年教师培养基金(晨光计划)(项目号:AASH2106)、上海建桥学院博士项目基金资助,向基金委员会致以感谢!
-
图 4 斜硅镁石样品的包裹体:(a-b)短柱状和长针状包裹体;(c-d)六方薄片状的固体包裹体;(e-f)无色的晶体包裹体和白色的絮状包裹体;(g-h)粒状黑色固体包裹体
Figure 4. Inclusions in clinohumite samples: (a-b)short columnar and long needle-shaped inclusions; (c-d)hexagonal sheet-like solid inclusions; (e-f)colourless crystal inclusions and white flocculent inclusions; (g-h)granular black solid inclusions
表 1 斜硅镁石样品的常规宝石学特征
Table 1 Conventional gemmological characteristics of clinohumite samples
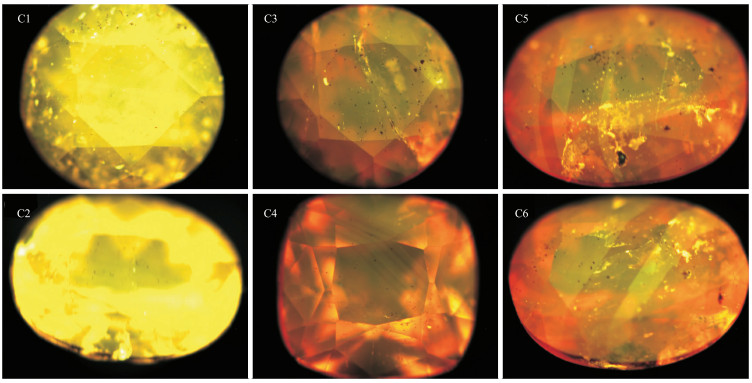
样品 颜色 光泽 折射率 双折射率 相对密度 紫外荧光 多色性 编号 LW SW C1 金黄色 玻璃光泽 1.629~1.660 0.031 3.14 惰性 中等黄色 中等—浅黄、黄色 C2 金黄色 玻璃光泽 1.629~1.660 0.031 3.22 惰性 中等黄色 中等—浅黄、黄色 C3 橙黄色 玻璃光泽 1.630~1.661 0.031 3.27 惰性 中等橙色 中等—黄色、橙红色 C4 橙黄色 玻璃光泽 1.632~1.668 0.036 3.20 惰性 中等橙色 中等—黄色、橙红色 C5 橙红色 玻璃光泽 1.632~1.668 0.036 3.23 惰性 中等橙色 中等—黄色、橙黄色、棕红色 C6 橙红色 玻璃光泽 1.633~1.668 0.035 3.23 惰性 中等橙色 中等—黄色、橙黄色、棕红色 表 2 斜硅镁石样品的化学成分定量分析
Table 2 Quantitative analysis of chemical compositions of clinohumite samples
氧化物 C1 C2 C3 C4 C5 C6 K2O/% 0.02 0.02 0.02 0.02 — 0.03 CaO/% 0.02 0.02 0.02 0.01 0.01 0.03 TiO2/% 2.13 1.93 2.36 2.76 2.63 2.63 MgO/% 55.74 56.86 55.75 55.18 56.03 55.02 SiO2/% 38.36 38.85 38.42 38.16 38.50 38.21 FeOT/% 0.06 0.05 0.27 0.22 0.22 0.22 Cr2O3/% 0.01 0.03 0.03 0.01 — 0.01 F/% 1.87 2.01 2.06 1.60 1.80 2.04 Total/% 97.43 98.93 98.05 97.27 98.44 97.34 元素阳离子个数 K 0.000 4 0.000 5 0.000 4 0.000 4 0 0.000 7 Ca 0.000 3 0.000 4 0.000 4 0.000 1 0.000 2 0.000 6 Ti 0.027 4 0.024 4 0.030 1 0.035 5 0.033 5 0.033 9 Mg 1.419 8 1.426 2 1.410 9 1.407 7 1.412 3 1.402 6 Fe 0.000 9 0.000 7 0.003 8 0.003 1 0.003 1 0.003 1 Cr 0.000 2 0.000 4 0.000 3 0.000 1 0 0.000 1 Si 0.655 3 0.653 6 0.652 1 0.653 0 0.650 9 0.653 5 N 2.210 9 2.222 6 2.217 3 2.215 9 2.226 3 2.205 1 注:表中N为M阳离子个数∶Si个数;不包括H2O;斜硅镁石中H2O质量分数的理论值为3%;FeOT包括FeO和Fe2O3;—表示低于检测限 -
[1] 梁祥济, 乔莉. 交代实验形成的硅镁石族矿物特征和物理化学条件[J]. 岩石矿物学杂志, 1990, 9(4): 340-350, 381. Liang X J, Qiao L. Characteristics of humite group minerals formed in metasomic experimentation and physico-chemical conditions for their formation[J]. Acta Petrologica et Mineralogica, 1990, 9(4): 340-350, 381. (in Chinese)
[2] 刘丹. 橄榄石和硅镁石族矿物的高温高压光谱研究及地质意义[D]. 武汉: 中国地质大学, 2023. Liu D. High-T and high-P spectroscopy study of olivine and humite minerals and its geological impication[D]. Wuhan: China University of Geosciences, 2023. (in Chinese)
[3] Miyake M, Nakamura H, Kojima H, et al. Cation ordering in Co-Mg olivine solid-solution series[J]. American Mineralogist, 1987(72): 594-598.
[4] Ottolini L, Camara F, Bigi S. An investigation of matrix effects in the analysis of fluorine in humite-group minerals by EMPA, SIMS, and SREF[J]. American Mineralogist, 2000(85): 89-102.
[5] Momma K, Izumi F. Vesta 3 for three-dimensional visualization of crystal, volumetric and morphology data[J]. Journal of Applied Crystallography, 2011(44): 1 272-1 276.
[6] 肖宇伦, 杨晓勇. 白云鄂博矿床一号碳酸岩脉铈磷灰石和硅镁石族矿物的矿物学特征及其地质意义[C]//中国矿物岩石地球化学学会矿床地球化学专业委员会. 第十届全国成矿理论与找矿方法学术讨论会论文摘要集, 2023: 3. Xiao Y L, Yang X Y. Mineralogical characteristics and geological significance of cerium apatite and humite group minerals in the No. 1 carbonate vein of the Baiyun Ebo deposit[C]// Chinese Society for Mineralogy, Petrology and Geochemistry. Summary of papers from the 10th National Symposium on Metallogenic Theory and Exploration Methods, 2023: 3. (in Chinese)
[7] 饶灿. 硅镁石族多体矿物的演化及其地质意义[C]//中国矿物岩石地球化学学会, 中国地质学会, 中国硅酸盐学会, 等. 2016年全国矿物科学与工程学术研讨会摘要集. 北京, 2016: 3. Yao C. The evolution and geological significance of multi body minerals in the humite group[C]// Chinese Society for Mineralogy, Petrology and Geochemistry, Geological Society of China, The Chinese Ceramic Society, et al. Summary of the 2016 National Symposium on Mineral Science and Engineering. Beijing, 2016: 3. (in Chinese)
[8] Carlos A R R, Oscar M C A, Carlos A C A. Ti-clinohumite in the Ciénaga skarn-type mineralogy, Sierra Nevada de Santa Marta Massif (Colombia): Occurrence and petrologic significance[J]. Earth Sciences Research Journal, 2015, 19(1): 15-30. doi: 10.15446/esrj.v19n1.42020
[9] Wunder B. Equilibrium experiments in the system MgO-SiO2-H2O (MSH): Stability fields of clinohumite-OH[Mg9Si4O16(OH)2], chondrodite-OH[Mg5Si2O8(OH)2]and phase A (Mg7Si2O8(OH)6)[J]. Contributions to Mineralogy and Petrology, 1998(132): 111-120.
[10] Lin C, Lin L, Mernagh T P, et al. Raman spectroscopic study of hydroxyl-clinohumite at various pressures and temperatures[J]. Physics and Chemistry of Minerals, 2000(27): 320-331.
[11] Lin D, Pang Y, Ye Y, et al. In-situ high-temperature vibrational spectra for synthetic and natural clinohumite: Implications for dense hydrous magnesium silicates in subduction zones[J]. American Mineralogist, 2019, 104(1): 53-63. doi: 10.2138/am-2019-6604
[12] Frost R L, Palmer S J, Bouzaid J M, et al. A Raman spectroscopic study of humite minerals[J]. Journal of Raman Spectroascopy, 2007, 38(1): 68-77. doi: 10.1002/jrs.1601
[13] Frost R L, Palmer S J, Reddy B J. Near-infrared and mid-IR spectroscopy of selected humite minerals[J]. Vibrational Spectroscopy, 2007(44): 154-161.
[14] Palmer S J, Reddy B J, Frost R L. Application of UV-Vis, near-infrared and mid-infraredspectroscopy to the study of Mn-bearing humites[J]. Polyhedron, 2007(26): 524-533.
[15] Reddy S L, Reddy N C, Reddy G S, et al. Characterization of clinohumite by selected spectroscopic methods[J]. Spectrochimica Acta Part A, 2006(65): 684-688.
[16] Clinohumite. Handbook of mineralogy[EB/OL]. [2024-04-17]. https://handbookofminearlogy.org/pdfs/clinohumite.pdf.
[17] Carty K, Cooper A. Inclusions in chondrodite[J]. Gems & Gemology, 2023, 59(1): 86-87.
[18] Kocman V, Rucklidge J. The crystal structure of a titaniferous clinohumite[J]. Canadian Mineralogist, 1973, 12(1): 39-45.
[19] Robinson K, Gibbs G V, Ribbe P H. The crystal structures of the humite minerals Ⅳ. Glinohumite and titanoclinohum[J]. American Mineralogist, 1973(58): 43-49.
[20] 谢鸿森, 彭文世, 薛承林, 等. 镁铁橄榄石系列的高压合成及其X射线衍射和红外光谱研究[J]. 矿物学报, 1986, 6(2): 103-108. Xie H S, Peng W S, Xue C L, et al. Synthesis, infrared spectra and X-ray diffraction of Mg-Fe olivine[J]. Acta Mineralogica Sinica, 1986, 6(2): 103-108. (in Chinese)
[21] Koike C, Chihara H, Tsuchiyama A, et al. Compositional dependence of infrared absorption spectra of crystalline silicate[J]. Astronomy and Astrophysics, 2003(3): 1 101-1 107.
[22] 闻骆. 矿物红外光谱学[M]. 重庆: 重庆出版社, 1989: 74. Wen L. Mineral infrared spectroscopy[M]. Chongqing: Chongqing Publishing & Media Co., Ltd., 1989: 74. (in Chinese)
[23] 陶隆凤, 苏慧慧, 郝楠楠, 等. 张家口及蛟河天然橄榄石的振动光谱研究[J]. 河北地质大学学报, 2022, 45(2): 52-55. Tao L F, Su H H, Hao N N, et al. Study on the vibrational spectra of peridot from Zhangjiakou and Jiaohe[J]. Journal of Hebei GEO University, 2022, 45(2): 52-55. (in Chinese)
[24] Seneewong M, Chongraktrakul W, Sripoonjan T. Gemological characterization of peridot from Pyaung-Gaung in Mogok, Myanmar[J]. Gems & Gemology, 2021, 57(4): 318-337.
[25] 薛纪越. 橄榄石-硅镁石多体系列晶格的系统畸变[J]. 矿物学报, 1987, 7(2): 97-106. Xue J Y. Systematic lattice distortion in the olivine-humite polysomatic series[J]. Acta Mineralogica Sinica, 1987, 7(2): 97-106. (in Chinese)
[26] 李晓静. 常见宝石的近红外光谱研究[D]. 昆明: 昆明理工大学, 2017. Li X J. Research on near infrared spectroscopy of common gemstones[D]. Kunming: Kunming University of Science and Technology, 2017. (in Chinese)
[27] Langer K, Platonov A, Matsyuk S S. The crystal chemistry of the humite minerals: Fe2+-Ti4+ charge transfer and structural allocation of Ti4+ in chondrodite and clinohumite[J]. European Journal of Mineralogy, 2002, 14(6): 1 027-1 032.
[28] Langer K. UV to NIR spectra of silicate minerals obtained by microscope spectrometry and their use in mineral thermody-namics and kinetics[M]//Salige E K H. Physical Properties and Thermodynamic Behaviour of Minerals. Dordrecht: Springer, 1988: 639-685.



 下载:
下载: